Author Archives: lpbrewer
Baltibrew General Meeting
October 18, 2018 @ Jon’s House
-
Bylaw Revisions Update
- Jon presented a list of several bylaw changes addressing the following areas:
- Officer Reductions
- Election Procedural Changes and Timeframes
- Quorum Requirements
- Greg requested that the language surrounding the club’s guild dues payment be modified so that the money comes directly from the club account.
- Jon presented a proposal to liquidate the club inventory due to lack of use and interest in maintaining it. The jockey boxes would default to Nepenthe as they put in the vast majority of time, labor, and maintenance costs for the upkeep of the boxes. Other items would be offered to club members.
- The specific language for the amendments presented at the meeting will be circulated to the membership and 2 votes will be held on the website for the charter changes and liquidation.
- Jon presented a list of several bylaw changes addressing the following areas:
-
Tasting Panels
- This will be the last year Jon will coordinate the tasting panels.
- The club may seek to continue doing pallet training as part of club events or education topics in the future.
- Following up on the previous motion/vote, the club reimbursed Jon $240 for the cost incurred in coordinating the event.
-
Club Calendar
- We expect planning to take place in November to map out club activities for 2019.
-
Officer Nominations
- Chilibrew Czar - Caiti was nominated and accepted on the condition that everyone is aware she and Jake may be relocation in the late spring. She will work to record all the information she can regarding Chilibrew logistics.
- Education Czar - Becky was nominated and accepted.
- Free State Homebrew Club Guild (FSHCG) Representative - Max was nominated and accepted.
- Meeting Czar - Jon was nominated and accepted.
- Social Secretary - Jude was nominated in absentia.
- Treasurer - Greg was nominated and accepted.
- Webmaster - Kris nominated himself.
-
Upcoming Events
- Mead Day on November 3rd @ Snyder’s Apiary
- Guild Holiday Party on Saturday, December 1st @ HS Brewery
Baltibrew secured a spot. Our theme will be farm oriented and will focus on the use of local/regional ingredients. Raj is coordinating and we need to know who will be volunteering to brew/cook for the table! Tickets are $25 dollars a head.
-
Iron Brewer IPA is on for December - Current Iron Brewer - The Reeds
-
November Meeting
- Jon will inquire about De Kliene
-
Homebrew Swap!
Baltibrew General Meeting
September 20, 2018 @ De Kleine Duivel
-
Introductions
-
Guild Update - Nate
- Christmas Party Charity Vote
The Guild is holding a vote for the beneficiary of their Christmas Party. Our vote is due by Sunday. We will post a poll on the website.
The Christmas party venue is still in flux. More information to come. Raj is interested in coordinating a Baltibrew theme/table. If anyone knows businesses that would be interested in donating items for the silent auction, let Nate know.
- Christmas Party Charity Vote
-
Bylaw Revisions Update
- Officer Reductions
The e-board is recommending eliminating the Secretary and Brewmaster positions and merging their duties into the other officer positions. The specific verbiage will be circulated for discussion. This change is mostly due to the reduction in interest in formal group brews and the recent active member count. - Election Timeline Changes
The e-board is recommending changing the officer term to coincide with the calendar year and streamlining the election process. This would move nominations to October, Stump Speeches to November, and elections/acceptance in December.
- Officer Reductions
-
Discussion of Baltibrew Events
- September 29th/30th - Sensory Panel Reschedule
Jon still has spots open for his tasting panels. This will be the last year he will coordinate them. Barrel flavors will be on the 29th, flaws & flavors on the 30th. The cost is $20 for 1 session, $35 for the pair. Greg recommended that Baltibrew reimburse any non-covered cost that Blair might incur as a result of the lack of interest. Jon will provide cost information for later action. - October 12th - Chilibrew XII!!!!
- This years beneficiary is the Teachers' Democracy Project (https://www.tdpbaltimore.org/).
- Competitor signup is closed.
- General Ticketing (https://chilibrew.yapsody.com/event/index/293111/chilibrew-xii)
- Volunteering - A volunteer ticker is available on the yapsody page. It’s a great way to attend for free and help out with this awesome event.
- Baltibrew Table - Kris will spin up an email thread to coordinate. We will try to run a new set of business cards to hand out.
- Anyone who knows a business that may be interested in donating items for the silent auction, get in touch with Jacob. The idea of donating some "learn to brew events" was suggested as a way for the club to encourage attendees to pick up the hobby.
- October General Meeting
As many people are wrapped up in beer week events and attendance in October is normally light, the meeting will be held at Jon’s house and be informal.
- September 29th/30th - Sensory Panel Reschedule
-
Social/Event Updates
- German Fest @ Max’s - September 22nd (https://www.facebook.com/events/1013482595477777/?notif_t=plan_user_invited¬if_id=1537553291207552)
- Zymernauts Crab Feast @ Goddard Space Flight Center - September 22 (https://maryland-homebrewers-guild.ticketleap.com/nasacrabs2018/)
- Midnight Homebrewers League present Carroll County Beer Week from September 24-29 (http://carrollbeerweek.com/)
- FOAM Presents Oktoberfest @ Frederick Fairgrounds on September 28 & 29th (http://www.frederickoktoberfest.org/)
- Maryland Microbrewery Festival @ Union Mills - September 29th (http://www.marylandmicrobreweryfestival.com/)
- Sapwood Cellars Grand-ish Opening - September 29th (https://www.facebook.com/events/1094587937371254/)
- Sour Class @ MDHB on Monday, October 15th 7-9 (www.mdhb.com)
- Das Best Oktoberfest w/ Homebrew Competition - Saturday, October, 13th
- Homebrew drop off - September 21st (www.mdhb.com)
- Beer Historian Rob Pattinson @ MDHB on Thursday, October 18th (www.mdhb.com)
- MALTs Turkeyshoot Competition, November 3rd @ Hysteria Brewing (https://turkeyshoot.brewcompetition.com/) - Entries due October 27th
- Mead Day on November 3rd @ Snyder’s Apiary
- Guild Holiday Party on Saturday, December 1st @ TBD
-
Other New Business/Member Announcements
- Nepenthe Brewery Update
Construction is continuing. The homebrew shop will be accepting online orders soon. The roof doesn’t leak and electricity has been run. They are shooting for a November opening!
- Nepenthe Brewery Update
-
Iron Brewer Call for Challengers - Current Iron Brewer - The Reeds
Jon challenged the Reeds to an IPA battle in December. Bring your entry!
-
Ed Topics
- Yeast Capture Update - Tasting!
5 different samples of Baltimore yeasts were distributed for tasting! Becky and Max’s capture from Riverside Park produced an extremely fruity aroma with a very clean taste. The Locust Point Flower capture yielded a mild-spicy phenolic profile. Anyone with tasting notes is encourage to email Kris! - Brut IPA Tasting!
- Baltimore Brewing History
Christophe is helping with a project to extract information from a book on the history of brewing in Baltimore. Watch out for more information.
- Yeast Capture Update - Tasting!
-
Homebrew Swap!
Which charity should receive the proceeds from the Guild Christmas Party?
- Dogs XL Rescue (50%, 4 Votes)
- Gallagher Services (25%, 2 Votes)
- Warrior Music Foundation (13%, 1 Votes)
- Maryland Center for Veterans Education and Training (13%, 1 Votes)
- Animal Advocates of Howard County (0%, 0 Votes)
- Cystic Fibrosis Foundation (0%, 0 Votes)
- Rise for Autism (0%, 0 Votes)
Total Voters: 8
As a reminder our September meeting will be at De Kleine Duivel next Thursday, September 20th @ 8PM!
Back when we started our journey to wrangle some wild local microorganisms the biggest question we faced was not if we could capture yeast, but if we could capture yeast that would make beer.
Now we find ourselves at a crucial juncture. We need to measure the attenuative properties of our yeast isolates to determine if any of the samples can ferment the more complex sugars in a malt based wort.
Yeast is everywhere in nature, living in places like flowers, fruit, and tree bark. It grows by converting sugars into the energy it needs to multiply, producing ethanol as one of its coveted byproducts. All yeast can consume simple sugars like sucrose, glucose, and fructose.
Over the centuries many cultures developed rich traditions of fermented food and beverage. Before humans discovered the organisms responsible and assigned them names, people had already modified these lifeforms by reusing material from pleasing results. Brewers yeast, with its ability to ferment the maltose that makes up the majority of wort sugars, has been under heavy selection pressure throughout the course of human history.

Saccharomyces cerevisiae, SEM image
The packs of dried and liquid yeast available to modern homebrewers are the culmination of years of brewing study and science. We are fortunate to be able to choose from a variety of proven yeast strains (and other organisms) when crafting our recipes.
The beers produced from this panel would have no such advantage.
Our project began in April when we prepared and distributed capture kits to our members.
In June, we triaged the results and jumped into the lab to plate and examine the captured organisms.
Baltibrewer Becky set to work this July, isolating and culturing up quantities of 6 strains of budding yeast. We decided the quickest way to find out what we had was conduct to a survey of 1 quart samples. The finished fermentations would be checked for specific gravity and pH drops. If any results were pleasing we’d bottle a 12 ounce sample. Yeast cakes would be saved for future use in larger batches.
Becky stepped up the cultures in the lab over the course of a few weeks and tested the samples for purity. On August 9th we met up with our yeast for a brewday!

Our yeast isolates!
For simplicity, we used Briess Light Dry Malt Extract and distilled water for our wort. We added a single charge of UK Sovereign hops for a bittering addition at 30 minutes.
| Original Gravity (Brix) | IBU | pH |
| 1.051 (13.2) | 20 | 5.26 |
While the wort chilled we sanitized jars and airlocks. Given the lack of headspace in our tiny fermenters we added a few drops of Ferm-Cap-S as we portioned the wort 7 ways (one extra jar for a US-05 control). We pitched our samples and to our pleasure within a day or two we were off to the races!

It begins!
Some jars showed more vigorous activity than others. We observed action in the airlocks of most. Given the homemade nature of our airlock fixture it was certainly possible our seals were not perfect. We decide to wait at least 2 weeks before checking the results. On August 29th, we gathered to investigate.

Getting down to the business of bottling.
We processed the beers one at time. A single jar was briefly opened and a sanitized pipette used to remove liquid for a Brix reading. After we assed attenuation, we moved on to a quick smell evaluation. Any interesting results were bottled and the finished pH noted. The rest of the sample would be transferred a smaller container and stored cold.
It was time! What would we find!
To our amazement, 5 of 6 samples showed a significant drop in gravity and pH! The threat of commercial contamination loomed in our heads as we recorded tasting notes from the uncarbonated beer.
To our pallets the cultures produced distinct flavor profiles in the fermented products, some quite notable! Between the variety of results, the different levels of activity and flocculation, and the fact that one culture did not attenuate well at all, we’re very hopeful these fermented beers represent true isolates from wild captures and our coolship barrels!
| Sample | Final Gravity (Brix) |
pH | Apparent Attenuation |
ABV | Notes |
| US-05 | 1.012 (6.6) | 4.07 | 76.4% | 5.25% | Did not save the yeast cake, tasted like a simple beer consistent with US-05 |
| Caiti | 1.013 (6.8) | 4.42 | 75.1% | 5.17% | Pleasing phenolic/spicy aroma, malty sweet taste |
| Riverside Park | 1.015 (7.5) | 4.43 | 70.9% | 4.88% | Fruity aroma, clean flavor profile with low esters |
| Kwanzan Cherry |
1.015 (7.5) | 4.40 | 70.9% | 4.88% | Pleasing but more assertive phenolic/spicy aroma, mild phenols on the front, with a mild dry/tannin finish |
| Little Barrel | 1.031 (11.2) | 4.69 | 40.0% | 2.80% | Did not bottle, smelled like baby diapers |
| Big Barrel (Dark Purple) |
1.016 (7.8) | 4.42 | 69.0% | 4.76% | Malty sweet aroma with phenols, sweet estery taste with more noticeable body |
| Big Barrel (Light Purple) |
1.014 (7.2) | 4.40 | 72.7% | 5.01% | Cinnamon/earthy spice aroma, sweet and dry taste with a peculiar spicy phenol during the finish |
We buzzed with excitement as we cleaned, dreaming of things we could do with the saved samples! But as we looked to the future, we also reflected on the road to this point. Capturing this many strains of yeast that could complete a fermentation was somewhat unexpected. How did we end up here?
Was our capture medium with its low OG, IBU, and pH a sufficient filter for the small amount of malt friendly yeast to take hold? Did the large number of initial captures followed by aggressive triage of moldy and failed samples set us up for a better success rate once we got into the lab? Were our expectations set too low to begin with? We’ll continue to reflect on the project as we wait to taste the carbonated samples at our next meeting.
With fall on the horizon we are eager to continue our quest to make beers with native strains of Baltimore yeast. Cheers and happy homebrewing!

A big milestone in our project: bottled beer and saved yeast from successful fermentations.
Baltibrew General Meeting
August 16, 2018 @ De Kleine Duivel
-
Introductions
-
501c7 Application/Bylaw Revisions Update
- Nothing more will happen on the 501c7 application in 2018. We will revisit this in 2019.
-
Discussion of Baltibrew Events
- September 8th - Fall BBQ & Brew @ Jacobs
The original event was scheduled for the holiday weekend so the event has been rescheduled to September 8th. - September 29th/30th - Sensory Panel
Seats are still available, contact Jon if interested. - October 12th - Chilibrew XII!!!!
- We have one sponsor so far and are seeking a few more. Anyone with a lead on an interested business should reach out to Jacob.
- Competitor Signup Open we have 17 so far and are hoping for about that many more.
- (https://chilibrew.yapsody.com/event/index/286756/chilibrew-xii-competitor-ticket)
- General Ticketing to follow.
- Volunteering is a great way to attend this event for free. Caiti will be the volunteer coordinator. Reach out to her if interested.
- Kris is going to chair the Baltibrew Table, if you are interested in brewing for the table, please reach out. We are looking for 4 brewers to rep our club at the event.
- September 8th - Fall BBQ & Brew @ Jacobs
-
Social/Event Updates - Jacob
- Maryland State Fair - Dropoff on Tuesday, August 21 and Wednesday, August 22 (www.mdhomebrewers.org)
- Zymernauts Crab Feast @ Goddard Space Flight Center - September 22 (https://maryland-homebrewers-guild.ticketleap.com/nasacrabs2018/)
-
Other New Business/Member Announcements
- Nepenthe Update - Things are as busy as ever! They are hoping to open their doors by November!
-
Iron Brewer IPA
- The Reeds took home the Iron Brewer trophy with their New England IPA! Congratulations!
- The Reeds took home the Iron Brewer trophy with their New England IPA! Congratulations!
-
Ed Topics
- Yeast Capture Update!
Becky grew up six yeast isolates from the group captures and they are currently fermenting in one-quart batches. We will take gravity and pH measurement next week to see how well these solo organisms ferment. - May I Have Your Attenuation, Please!
A discussion on attenuation and the new Brut IPA style.
- Yeast Capture Update!
-
Homebrew Swap!
Baltibrew General Meeting
July 19, 2018 @ Nepenthe
-
Introductions
-
501c7 Application/Bylaw Revisions Update
- No new news on the 501c7 application as yet.
-
Discussion of Baltibrew Events
- Sensory Panel Update - Jon
Seats still available. Contact Jon if you are interested. - August 4th - August Brewery Trip - Greg/Jacob
- August 16th-18th - MASHOUT
- September 1st - Fall BBQ & Brew @ Jacobs
- October 12th - Chilibrew XII!!!!
If you are interested in brewing for the ChiliBrew table contact Carian.
- Sensory Panel Update - Jon
-
Social/Event Updates - Jacob
- MDHB Beer Education Classes
- Practical Sour Beers class - Tonsmeire ($35), July 21st (http://mdhb.com/product_info.php?products_id=9008)
- Recipe Formulation ($10), July 22nd - Email Les
- Barkhappy Summer Pawty @ Monument City to benefit the Baltimore Human Society - July 28th (https://www.facebook.com/events/244860942933479/)
- Union Collective 6th Celebration & Grand Opening - July 28th
- Hopfest @ Max’s - August 9th (https://www.facebook.com/events/2014185921939526/)
- Catonsville Coop’s Hometown Brew Down - August 11th (https://www.catonsvillecoop.com/calendar/2018/8/11/5th-annual-hometown-brewdown)
- Zymernauts Crab Feast @ Goddard Space Flight Center - September 22
- MDHB Beer Education Classes
-
Other New Business/Member Announcements
- Thank you to Nepenthe!
- Upcoming Monthly Meeting Locations
- August & September - de Kleine Duivel
-
Iron Brewer Call for Challengers - Current Iron Brewer - Will
-
Homebrew Swap!
This weekends sensory tasting panels have been postponed. New dates will be circulated once the event is rescheduled.
In April we distributed homemade yeast capture kits to the club at our monthly meeting. Our goal? To capture and isolate a strain of yeast native to Baltimore and use it to make a fermented beverage!
Members were eager to try their luck. The weather was poised to cooperate. Lows would bounce between 40 & 50 F for the next few weeks, a range said to be helpful when harvesting wild yeasts.
Things were looking up as we grabbed flowers from our backyards & local parks, swabbed out barrels used for coolshipped wort, or left our covered jars tucked safety outside. All that was left for us to do was to sit back and wait for the airlocks to start bubbling. So we waited!
And waited.
And waited.....
Several members saw no activity at all. Others observed growth of mold or other non-yeast organisms.

This doesn't look too good.
As the reports came in it was clear that we were not going to see widespread examples of obvious & vigorous fermentation. So we began to triage the captures. Anything clearly growing mold was disposed of. The few samples that did report airlock activity were shipped off for early plating. The coolship barrel swabs proved more promising and were left to themselves for a month before streaking. When all was said and done the club selected 4 captures from a total of 16 trials:
- Open Air Capture - Riverside Park
- Kwanzan Cherry Flower - Locust Point
- Coolship Barrel #1 (Big Barrel) - Pikesville
- Coolship Barrel #2 (Little Barrel) - Pikesville
While not the quantity we were hoping for it was nevertheless time to move this party into...
The Laboratory
We're very grateful to Baltibrewer Becky who is doing the detail work on this project. Her first step, check the gravity (all samples stared at a Brix of 6.4 or 1.025 SG):
- Riverside Park - 4.4 Brix/1.017 SG
- Kwanzan Cherry - Negligible change
- Big Barrel - Negligible change
- Little Barrel - 4.2 Brix/1.017 SG
We found it interesting that two samples did not register a gravity drop. They both showed airlock activity, became turbid, and accumulated a layer of sediment on the bottom as activity slowed.
Did we incorrectly measure the first Brix reading? Was there really no decrease in gravity?

A happy capture?
Cultures taken from the samples would tell us more. One drop of medium was transferred to individual Sabouraud Dextrose (Sab) plates. Plates were streaked for isolation and incubated in the dark at room temperature (approximately 68 degrees F). Within days they showed abundant growth.

Open Air (Riverside Park) Plate
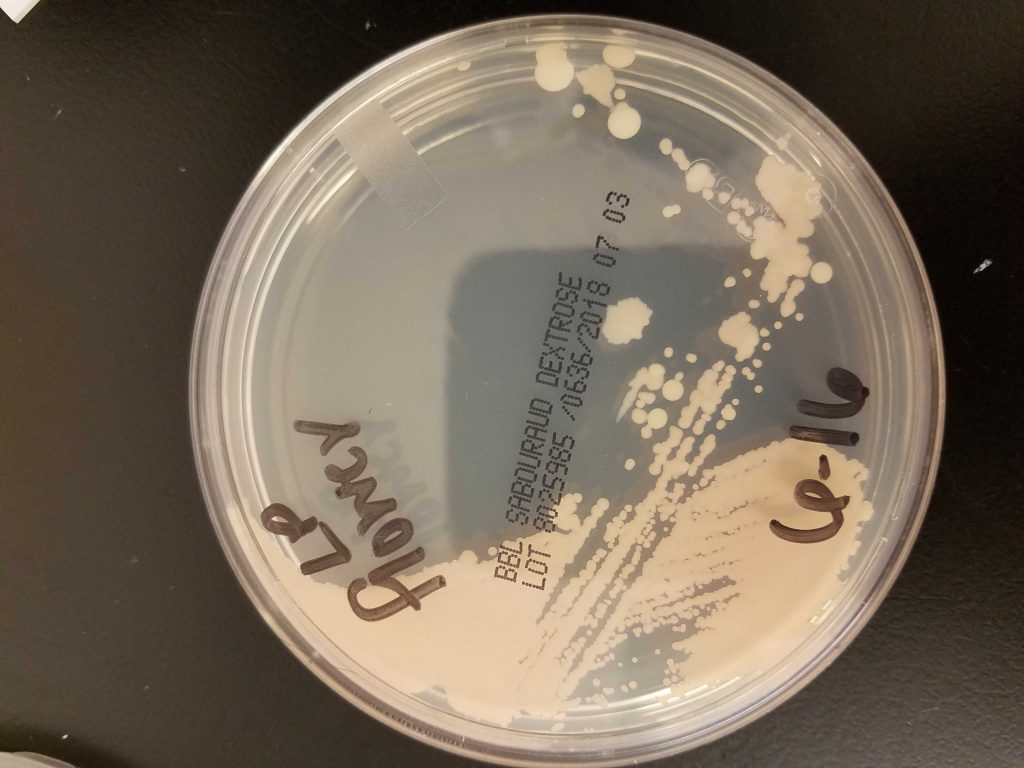
Kwanzan Cherry Plate
Based on morphology the following organism were identified:
- Riverside Park - 1 budding yeast & 2 types of motile bacteria
- Kwanzan Cherry - 1 budding yeast & 2 bacteria morphologies
- Big Barrel - 2 types budding yeasts & a bacteria
- Little Barrel - 1 apparent pure yeast strain
Success from failure? Possibly.
While the yeast strains are isolated and grown up, it's worth considering what we've accomplished and where we might go from here. The original plan was to isolate native organisms for use in a malt based fermented beverage. We now have at least 2 wild Baltimore yeasts on our plates and 3 more selected from barrels used after coolshipping. Two showed at least moderate attenuative properties in the capture media. Several did not.
Trials will tell us more but it is fair to suspect that these yeasts may not do well fermenting a malt based wort on their own. So is our plan still viable?
Michael Tonsmeire of The Mad Fermentationist blog and Sapwood Cellars (opening soon) stopped by our June meeting to share funky beers and talk about his brewing philosophies. One topic he spoke on was recognizing and embracing your individual strengths and scales.
As homebrewers it can be hard to make an Octoberfest that nails the style as well as commercial brewers. They have first pick of malts, laboratories to grow up strong & pure pitches of yeast, rigorous fermentation & packaging controls, and employees devoted entirely to quality control & sensory evaluation.
On the other hand we are not beholden to the demands of bulk production. This affords us flexibility. For example, we can experiment with secondary additions from local crops, using fruits that aren't grown in large enough quantities to be an option for commercial offerings. Just last month Mulberry Trees in our area started to yield ripe fruit. For anyone not familiar the mulberry is a delicious, mildly sweet & jammy tasting fruit that looks a lot like a blackberry.

Mulberry Fruit & Leaves (Andre Abrahami, May 28th, 2007)
Perhaps we won't end up with a yeast that can make a beer. But perhaps we will end up with several capable of producing local fruit wines and ciders! We will stick to our strengths as homebrewers and stay nimble. Cheers and happy brewing!
Read more as we take our isolates out of the lab for a test drive!
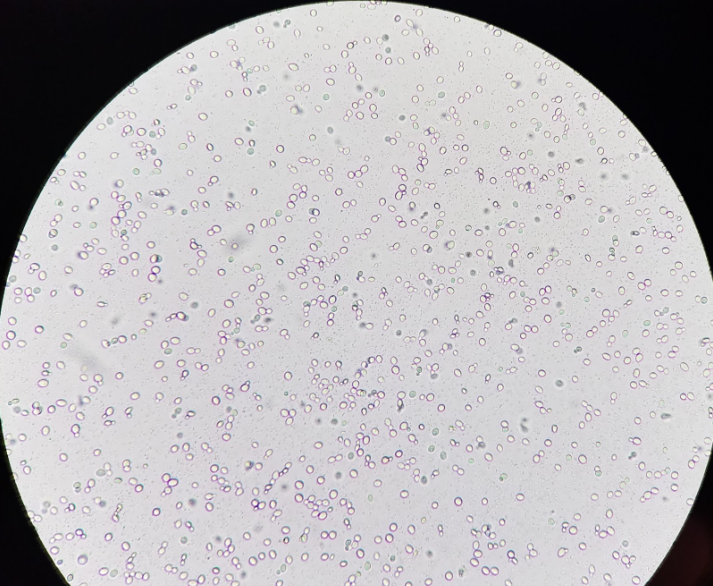
Budding Yeast & Motile Bacteria
Baltibrew General Meeting
June 21, 2018 @ Nepenthe
-
Introductions
-
501c7 Application/Bylaw Revisions Update
-
Our application for 501c7 status was denied by the IRS due to the gross receipts from our charity fundraiser. Greg has reached out to Maryland Nonprofits for guidance and we are waiting to hear back.
-
-
Discussion of Baltibrew Events
-
July 14th/15th - Sensory Classes @ Jon's
Seats are still available. Day 1 will focus on barrel aging flavors. Day 2 will focus on common flavors and faults. 20$ for one session, $35 for two. Talk to Jon to sign up. -
July 28th - Union Collective Opening
-
August 4th - August Brewery Trip
The current plan is to do a local trip, starting at Key around noon the relocating to the Mount Vernon area in the early afternoon to the area of Brew House 16, Charm City Meadworks, The Brewer’s Art, The Brass Tap, & Wet City. We’re exploring options to encourage ridesharing, possibly making a Lyft or Uber credit available to folks who share cars. More to come. -
September 1st - Fall BBQ & Brew @ Jacobs
-
-
Social/Event Updates
-
Annapolis Homebrew Club’s Pints for Paws Homebrew and Craft Beer festival, June 22nd (https://www.eventbrite.com/e/
4th-annual-pints-for-paws- homebrewing-and-craft-beer- festival-tickets-44105596025) -
MDHB Beer Education Classes
-
Recipe Formulation ($10), July 22nd
-
Mike Tonsmeire will also be hosting a session at MDHB in July.
-
-
-
Other New Business/Member Announcements
-
We will have alternate meeting locations later this summer as Nepenthe is moving to their new space. We will meet at the shop one last time in July. The August meeting is TBD and the September meeting is confirmed for De Kleine Duivel.
-
-
Iron Brewer Call for Challengers - Current Iron Brewer - Will
-
Ed Updates
-
Water Wrap Up!
A couple of us visited the Ashburton water treatment plant for a tour. It’s an amazing operation and the staff was very friendly and informative. One items to pass on is that as far as the laboratory chemist was aware there are no changes the chemical protocols when the city pulls from the Susquehanna and the city does not use chloramines to treat the water at this time. We may drop in on the older Montebello plants. -
Baltibrew Yeast Capture
Becky has a few captures plated out in the labs and has detected at least one strain of budding yeast that is likely Saccharomyces. We’re not sure about the attenuation properties of any of the organisms. A back up plan to do a local fruit wine is in the works should we strike out on organisms that can ferment malt.
-
-
Ed Topic - Sour Hour feat/ Michael Tonsmeire (Sapwood Cellars, The Mad Fermentationist, American Sour Beer)!
-
Homebrew Swap!

